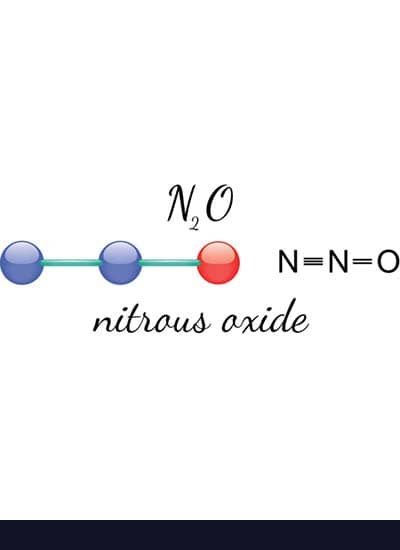
What Is Nitrous Oxide?
Nitrous oxide (N2O), also known as laughing gas, is a colorless, sweet-smelling, sweet-tasting gas that is used in dentistry and surgery because inhaling the gas produces analgesic and anesthetic effects. The gas is also used to improve the engine output of automotive vehicles and as an oxidizer in rocketry. Nitrous oxide is called “laughing gas” because inhaling it produces euphoria.
How to Make N2O? (How to Make Nitrous Oxide)

- The manufacturing process for N2O is relatively simple. The raw ingredient, ammonium nitrate (NH4NO3), is supplied as a clear liquid (liquid ammonium nitrate, LAN) or as solid, pellet-sized particles (solid ammonium nitrate, SAN). This material is used for fertilizer and as a primary ingredient of explosives.
- To make N2O, NH4NO3 is heated to approximately 250° C. At that point, the NH4NO3 decomposes into N2O, water (steam), and some contaminants (NH4NO3 →N2O + 2H2O). The gas mixture is cooled to ambient room temperature, the steam is condensed, and most of the water is removed. The resulting crude N2O gas mixture is scrubbed to extract the contaminants. The gas is then compressed, dried to remove the remaining water, cooled, and liquefied. The resulting product is nearly pure (99.5% to 99.9%) and is stored as a liquefied, compressed gas at approximately 300 psi and 0° to 10° F in insulated and refrigerated storage tanks.1 Figure 6-1 is a flowchart of the steps involved in manufacturing, repackaging, and distributing N2O.
How Is Laughing Gas Used?
Laughing gas is an anesthetic, medical professionals use to keep patients calm before surgery or other procedures without fully sedating them.
Your doctor may ask for your consent prior to your procedure. The plastic mask will then be placed on your mouth and nose. The laughing gases flow through the plastic mask as you breathe them in.
Nitrous oxide is a depressant, so it slows your body down. Once it kicks in, you may feel:
- Happy
- Giggly
- Light-headed
- Mild euphoria
- Relaxed









No comment